ABOUT ASTRUM-005

ASTRUM-005: ONE OF THE LARGEST INTERNATIONAL TRIAL IN ES-SCLC1
ASTRUM-005 is an international, double-blind, phase 3 randomized clinical trial conducted at 114 hospital sites in 6 countries (China, Georgia, Poland, Russia, Turkey, Ukraine).
The objective of the study was to evaluate the efficacy and adverse event profile of the PD-1 inhibitor serplulimab plus chemotherapy compared with placebo plus chemotherapy as first-line treatment in patients with extensive-stage SCLC.
585
patients were randomized 2:1 to the serplulimab plus chemotherapy group or placebo plus chemotherapy group
Key inclusion criteria:
- Histologically or cytologically confirmed ES-SCLC
- No prior systemic therapy for ES-SCLC
- 1 or more measurable lesions assessed using RECIST version 1.1
- An Eastern Cooperative Oncology Group Performance Status (ECOG PS) Scale score of 0 or 1
- Adequate organ function and life expectancy of 12 weeks or longer
Key exclusion criteria:
- Mixed-stage SCLC
- Active central nervous system metastases or carcinomatous meningitis
- Autoimmune disease
Interventions:
- Patients received either 4.5 mg/kg of serplulimab or placebo via intravenous infusions every 3 weeks until disease progression, death, unacceptable toxicity, withdrawal of consent, or other reasons specified in the trial protocol
- All patients received 100 mg/m2 of etoposide on days 1, 2, and 3 and carboplatin within the area under the serum drug concentration time curve of 5 mg/mL/min (up to 750 mg) on day 1 of each cycle for up to 4 cycles via intravenous infusions
Kaplan-Meier estimate of overall survival
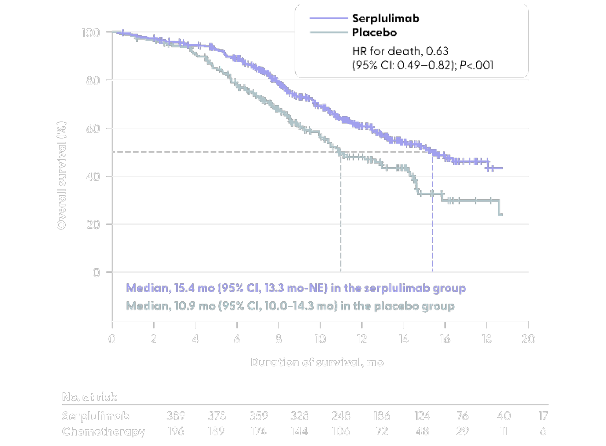

Data show the Kaplan-Meier estimates of overall survival in ASTRUM-005 randomized phase 3 clinical trial evaluating the efficacy of first-line serplulimab versus placebo added to chemotherapy in ES-SCLC patients. Patients who received serplulimab demonstrated
- Median overall survival of 15.4 months (95% CI, 13.3 months-not evaluable) in the serplulimab group compared with 10.9 months (95% CI, 10.0-14.3 months) in the placebo group
WHY ASTRUM-005 MATTERS
While serplulimab is not approved in the US, the ASTRUM-005 trial provides research that may hold great promise for ES-SCLC patients.
The trial results showed that patients with PD-L1 + previously untreated ES-SCLC who were given serplulimab plus chemotherapy had significantly improved overall survival compared with chemotherapy alone.
Serplulimab is an investigational therapy in the US and is not FDA approved. The ASTRUM-005 trial provides important research on its potential treatment for ES-SCLC.

ECOG PS=Eastern Cooperative Oncology Group Performance Status; ES-SCLC=extensive-stage small cell lung cancer; PD-1=programmed cell death 1; PD-L1=programmed cell death ligand 1; RECIST=Responsive Evaluation Criteria in Solid Tumors; SCLC=small cell lung cancer.
Reference: 1. Cheng Y, Han L, Wu L, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. JAMA. 2022;328(12):1223-1232. doi:10.1001/jama.2022.16464


