DISCOVER ASTRIDE
ASTRIDE: BRIDGING RESEARCH AND INNOVATION
THE ROLE OF BRIDGING TRIALS
A bridging study is designed to support the extrapolation of foreign clinical data—including safety, efficacy, dosage or dose regimen—to a new region and regulatory body. Data from a bridging trial can help satisfy the US Food and Drug Administration (FDA) requirements for the acceptability of foreign data for marketing approval.1,2
Today, new ES-SCLC therapies are available in China, Southeast Asia, and the European Union that are not approved for use in the US.3 A bridging study, ASTRIDE, has been initiated to explore the applicability of the data from the ASTRUM-005 trial for patients in the US.4
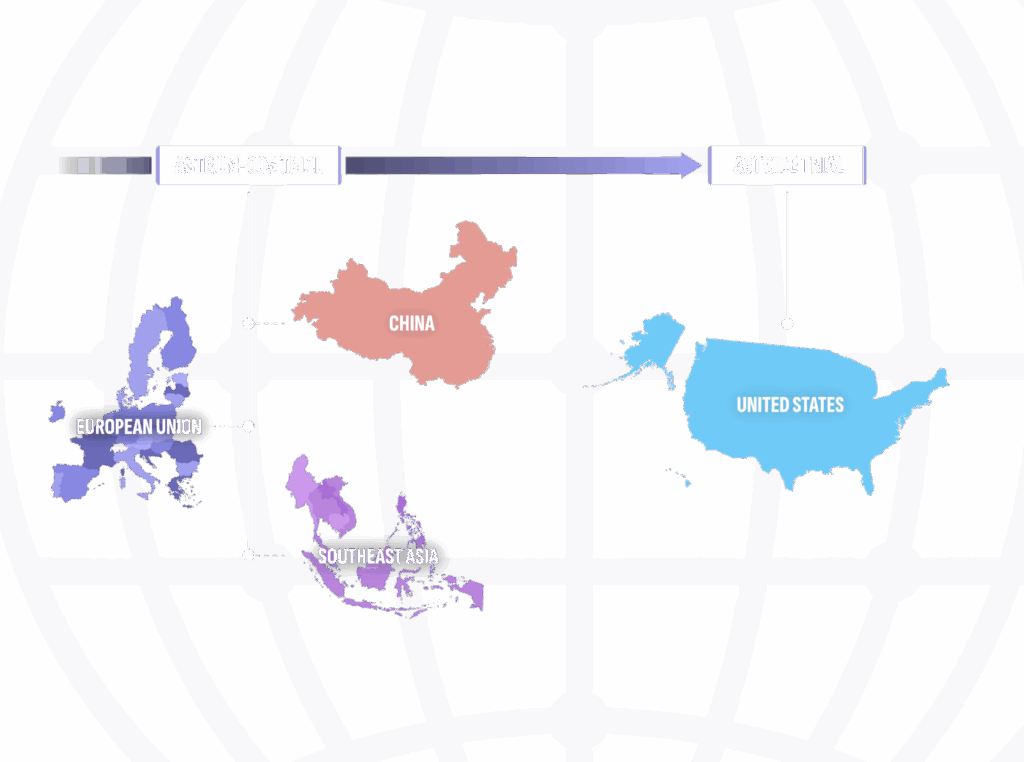

ASTRIDE: A UNIQUE BRIDGING STUDY, DESCRIPTIVE TRIAL COMPARING PD-1 VERSUS PD-L1 INHIBITORS4
BUILDING ON RESULTS FROM THE INTERNATIONAL PHASE 3 ASTRUM-005 TRIAL
Fosun has initiated this complementary registrational trial to confirm the applicability of the results from ASTRUM-005 to ES-SCLC patients in the United States.
LEARN HOW THE ASTRIDE TRIAL STRIVES FOR INCLUSIVITY
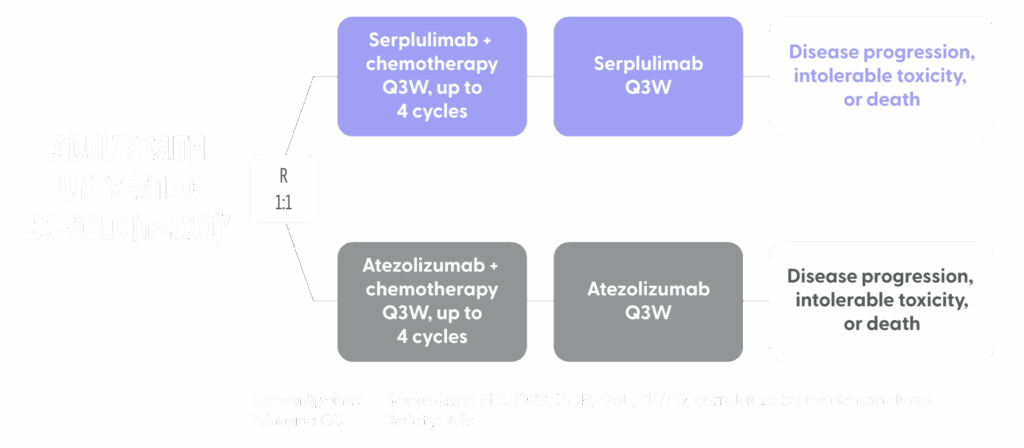
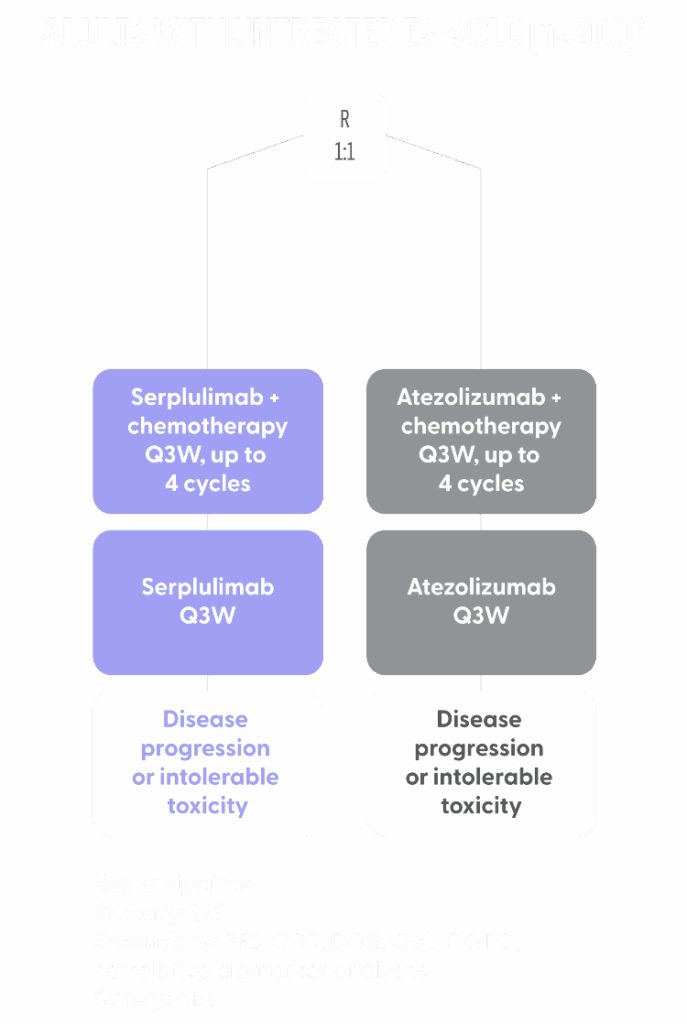
ASTRIDE TRIAL DESIGN
- ASTRIDE is a randomized open-label study of serplulimab + chemotherapy compared with atezolizumab plus chemotherapy in previously untreated patients in the United States with ES-SCLC4
- Atezolizumab was selected as the comparator arm because it is approved for first-line treatment of ES-SCLC in combination with chemotherapy and is considered a current standard of care in the US5
- Retaining atezolizumab as a comparator arm provides an important opportunity to observe treatment activity with PD-1 and PD-L1 side-by-side in the same trial6
- Key inclusion criteria7:


- Patients are randomized 1:1 to receive serplulimab or atezolizumab intravenously every 3 weeks until disease progression, intolerable toxicity, or death. All patients receive chemotherapy with intravenous etoposide and carboplatin every 3 weeks for up to 4 cycles7
Diversity Plan
- White
- African American
- Other
Exclusion
- Histologically or cytologically confirmed mixed-stage SCLC
- Prior systemic SCLC treatments
- Grade ≥2 peripheral neuropathy
- Ejection fraction <50% or NYHA class III to IV cardiac insufficiency
- Pregnant or breastfeeding females
FOSUN IS COMMITTED TO ADDRESSING HEALTH DISPARITIES AND IMPROVE HEALTH EQUITY
- The ASTRIDE trial inclusion and recruitment policy has been designed to be inclusive of underserved communities and to reflect the diverse population in the U.S.7
- Patient population:
- As enrollment continues, ASTRIDE will aim to include a higher percentage of minority patients in proportion to Caucasian patients to ensure a more equitable representation and to better understand serplulimab’s effectiveness across different demographic groups
- With specific patient demographics in mind, the ASTRIDE trial recruitment was designed to target patients who are most susceptible to lung cancer8

AEs=adverse events; ES-SCLC=extensive-stage small cell lung cancer; ORR=objective response rate; OS=overall survival; PD-1=programmed cell death 1; PD-L1=programmed cell death ligand 1; PFS=progression-free survival; PK=pharmacokinetic; QoL=quality of life.
References: 1. Code of Federal Regulations. 314.106 Foreign data. Accessed July 29, 2025. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part314/subpart-D/section-314.106. 2. Wang T, Cao X, He Y, Chen X. Innovation drug approvals based on a bridging study: from concept to practice. Transl Breast Cancer Res. 2022;3:2. Published 2022 Jan 31. doi:10.21037/tbcr-21-43. 3. Henlius. Henlius enters exclusive license agreement with Lotus for anti-PD-1 mAb serplulimab in South Korea. Accessed July 29, 2025. https://www.henlius.com/en/NewsDetails-4981-26.html. 4. Clinicaltrials.gov. NCT05468489. Accessed July 29, 2025. 5. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Small Cell Lung Cancer V.4.2025. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed July 29, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 6. Cheng Y, Han L, Wu L, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. JAMA. 2022;328(12):1223-1232. 7. Dowlati A, Wu E, Puri S, et al. Serplulimab vs atezolizumab added to chemotherapy in patients with treatment-naive ES-SCLC in the United States – ASTRIDE trial. Post in progress. 8. Wang Q, Gümüş ZH, Colarossi C, et al. SCLC epidemiology, risk factors, genetic susceptibility, molecular pathology, screening, and early detection. J Thorac Oncol. 2023;18(1)31-46.


